Nearly half of all pharmaceutical companies state they are poor or ineffective at using data to predict and resolve supply chain network shortages;1 putting access to medicine at risk and eroding margins. At the same time, many companies recognize that digital transformation is the path to address this.
When evaluating a digitalization strategy, a key objective is to identify software tools that enable more efficient and effective execution of tasks and business processes in a way that better connects people and processes. This is the difference between solving an isolated use case versus creating an enterprise-wide digital architecture that will be a sustainable differentiated advantage and is a core principle in achieving the Pharma 4.0 operating model.
AspenTech provides software solutions that accelerate digitalization for several key business functions, including modernizing the value chain. As the next step in the journey, the new aspenONE V14® release of the Supply Chain Management (SCM) software enables the communication of manufacturing and supply chain data across both internal and external networks of stakeholders for improved resiliency.
Align the Plant through Real-time Sitewide Communication
As discussed in a recent AspenTech blog, the Aspen Schedule Explorer™ supply chain software is a digital manufacturing collaboration hub that enables alignment between internal people and processes. Production schedules from Aspen Plant Scheduler™ are published to the commonly accessible web-based interface of Aspen Schedule Explorer. Stakeholders across the manufacturing plant can access and interact with the schedule, view current and past plans, communicate in a contextualized manner around specific batches and report on relevant data. Another recent blog highlighted how this digital manufacturing collaboration hub was key to enabling a batch production facility in Germany to increase its production throughput by 20% without incurring additional capital expenses.
Align the Network through Real-time Global Communication
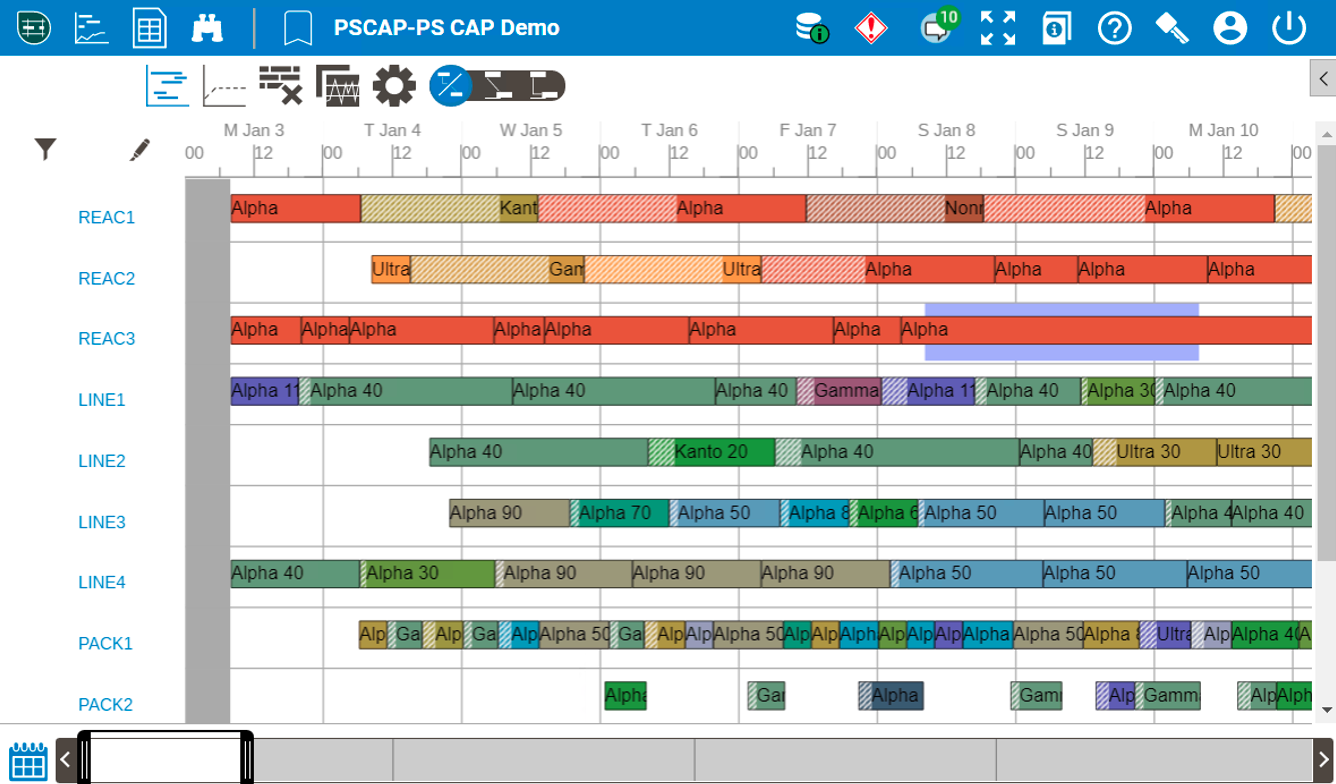
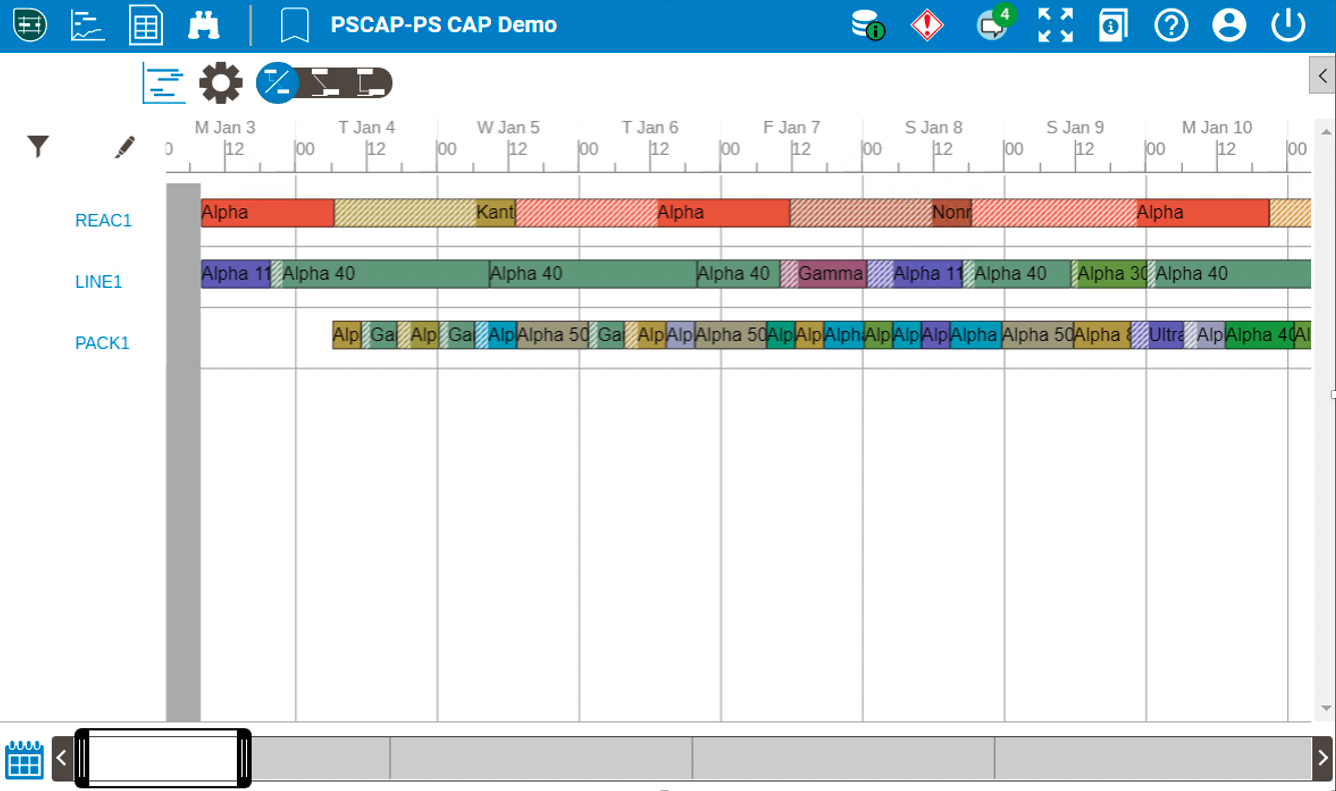
Internal plant alignment is not sufficient, this alignment needs to propagate across the supply network to achieve resiliency. Recent enhancements to the Aspen Schedule Explorer software allow for the external sharing of scheduling and manufacturing data. Permissioned visibility enables other sites in the pharma supply chain network, or even external contract manufacturing partners (CMOs), to view a subset of the production schedule including specific demands, facilities/equipment, materials and orders. This software functionality allows for planned schedules and actual manufacturing execution to be viewable across the globe in real-time. In addition to viewing, the external parties can communicate in a contextualized manner around specific batches, thereby improving synchronicity between historically inharmonious nodes in the network.
 |
 |
| Fig 1. Unrestricted View of Manufacturing Schedule |
Fig 2. Restricted View of Manufacturing Schedule |
Proactive Response through Enhanced Visibility
Fostering the connection between upstream manufacturing and downstream consumption, especially between external supply network nodes, will improve agility in response to process disruptions or material delays, the two greatest threats to the pharma supply chain and manufacturing networks2. For example, if a schedule is delayed due to an unforeseen manufacturing issue, the updated timeline is communicated to downstream stakeholders as the delay occurs. This proactive approach to disruption allows the nodes in the pharma supply chain network to stay in alignment and avoid costly downtime due to material issues.
Improving Pharmaceutical Supply Chain Resiliency
Better management of disruptions and communication of timeline changes will improve predictability of material flow and supply chain resiliency. The impact is a reduction in frequency and length of pharma supply chain shortages ensuring patients have reliable access to medicines. Furthermore, supply chain predictability allows companies to reevaluate the need for holding excess strategic inventories. Reduction in inventory has the potential to save pharmaceutical companies millions of dollars annually in material holding costs, further enhancing the ROI on supply chain software.
With any digitalization initiative, there are fringe benefits that can even outweigh the value of satisfying the primary use case. If software is evaluated under the lens of how well people and processes are connected, this software will provide compelling benefits and unlock hidden value for years to come.
- “Culture Reimagined - How Pharmaceutical Firms Can Use Data and AI with Confidence.” AspenTech, https://www.aspentech.com/en/resources/report/culture-reimagined-how-pharmaceutical-firms-can-use-data-and-ai-with-confidence.
- “The Needle Is Moving - Digital Transformation in Life-Sciences Manufacturing.” AspenTech, Axendia, 2022, https://www.aspentech.com/en/resources/report/the-needle-is-moving---digital-transformation-in-life-sciences-manufacturing.




/97.png?h=415&w=675&la=en&hash=5357C8331FC1998ABBC177D54C5673D3)
Leave A Comment