A key challenge in Life Sciences is to accelerate the pace of new product development while keeping costs down. As the product lifecycle progresses from discovery, through clinical trials to manufacturing scale-up, different teams often use different process representations to support their design decisions. This can fragment communication between teams, causing a lack of clarity around important assumptions and observations, which makes the final process design more costly and less robust. Limited equipment availability for experimentation, time consuming screening of new processes, siloed data and lack of visibility into scheduling options can all act as barriers to effective scale-up.
In response, digitalization of tech transfer - including data integration and common model frameworks for chemists, process development engineers and manufacturing engineers - delivers great value by fostering a shared understanding across teams of the interplay between design decisions around process recipe, materials choice and equipment selection. The understanding and accuracy gained enables process designs that are efficient, safe and trustworthy.
Aspen Batch Process Developer™ (ABPD) is a recipe-based modelling and simulation technology used for developing batch processes and generating required documentation — from early chemistry design to scale-up, technology transfer and production. ABPD facilitates the sharing of information across teams by providing a standard approach for creating and managing process information throughout the batch process development lifecycle. As a result, ABPD increases productivity, facilitates communication, and improves profitability by providing insights into operational changes and plant capacity debottlenecking.
Figure 1 shows the step-by-step recipe editor used to define the batch procedures in ABPD.
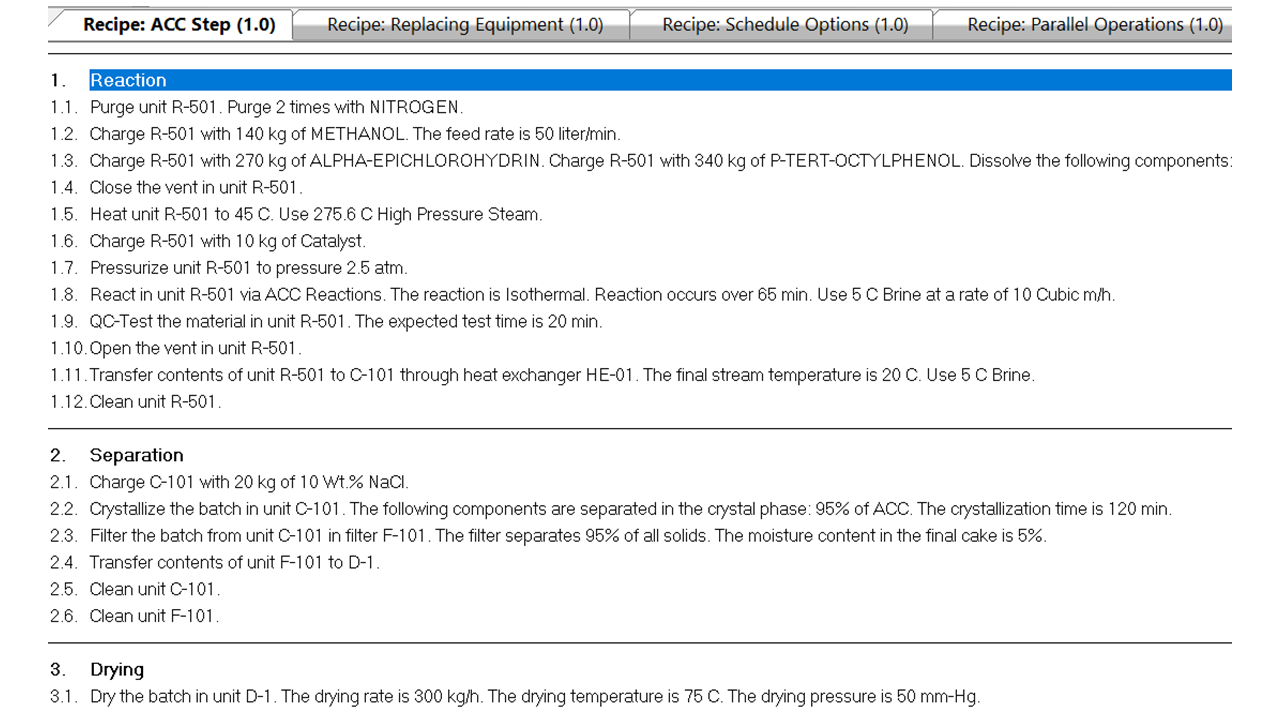 |
| Figure 1. Step-by-step recipe input. |
ABPD drives benefits for all the departments involved in the process development lifecycle – for both pharmaceuticals and biopharmaceuticals. Starting with the laboratory teams, the technology allows the comparison of process alternatives and selection of synthetic routes for new molecules. Progressing along the development lifecycle, process development engineers can screen new designs faster during the early stages of process definition to identify the configurations that are most cost effective, as well as evaluate emissions, waste generation and utility loads in order to decrease energy usage and excess solvent utilization, a boost for sustainability. This simulation-based approach improves asset utilization and workflow efficiency during the pilot plant stage. In addition, it helps accelerate process scale-up and allows better planning of capacity expansion for production at commercial scale. Beyond the needs of tech transfer, this approach also delivers a validated process model that can be employed to troubleshoot problem batches during future commercial production.
Figure 2 shows the batch schedule, with time profiles for equipment and services utilization.
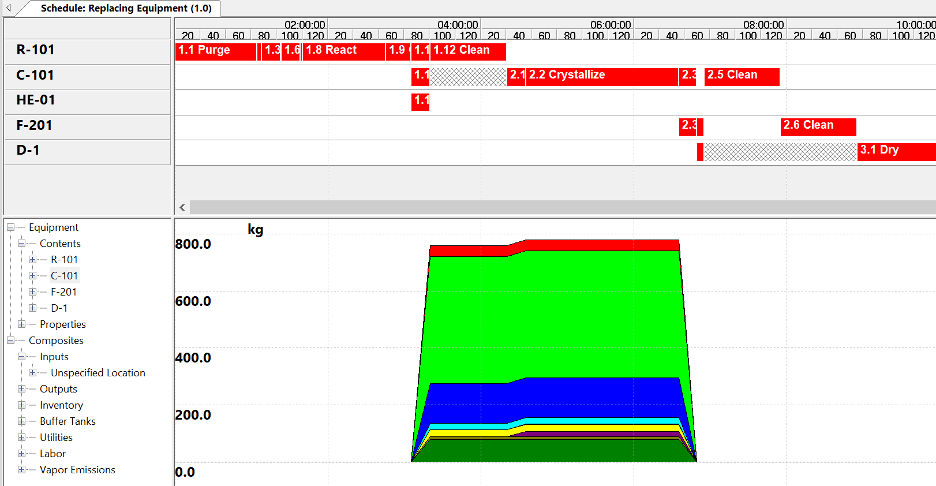 |
| Figure 2. Visual batch schedule: Equipment/services utilization and key data. |
ABPD is empowered by other solutions in the aspenONE® portfolio, such as Aspen Properties®, which is the industry standard for thermophysical properties modeling, relying on an extensive component and properties database, including state-of-the-art activity coefficient models and equations of state. Other integrations are solubility analysis for solvent selection and the Aspen Properties Microsoft Excel add-in for deploying calculation tools.
Achieve a common language, understanding and workflow between teams in your product commercialization life cycle with the insights and synergies provided by ABPD.
Learn more about how to optimize your digital transformation journey with AspenTech solutions for Pharmaceuticals by visiting www.aspentech.com/pharma.
.png?h=250&w=975&la=en&hash=7BEDB5E18E6803433F6D2D7630B4F5AD)

.png?h=415&w=675&la=en&hash=85E67E35726B6CE52927DA8A1D281768)



Leave A Comment