The pharma and biopharma landscape continues to transition from blockbuster drugs to increasingly niche medicines. An effective response, by both innovators and contract manufacturing organizations (CMOs) alike, depends on advances in cost- and time-efficient methods for designing and refining production platforms for new medicines — whether batch or continuous. This demands the design space be quickly mapped to find both design and operating conditions which are simultaneously robust and economically viable. Given the pressures on time and materials, wide-ranging studies based on physical experiments are no longer optimal.
Digital twins can help overcome those challenges. Essentially a digital replica of the design space, a digital twin allows companies to run a comprehensive set of virtual experiments. This enables a focused set of physical experiments to be chosen that efficiently utilize time and materials to fine-tune the design for optimum yield and quality assurance. The result is faster technology transfer, the hand-over of a process from design to manufacturing or to a CMO/CDMO, helping to maximize revenue potential under patent protection. Technology transfer is complicated, time-consuming, and costly. It’s estimated that a company loses 80% market share when a patent expires, which means each additional day spent on tech transfer can mean millions of dollars in lost revenue.1 In addition to accelerating technology transfer, this approach can be extended to continued process verification (CPV) to ensure reliable production, with quality assurance during the product life cycle.
The assurance provided by Quality by Design (QbD) generally demands substantial amounts of supporting data, which is a significant resource challenge. Enter hybrid modeling, a technology that combines mechanistic and data-driven approaches to merge the strengths of first principles understanding with real-world, data-driven verification. The hybrid approach is a valuable contributor to accelerate effective mapping of the design space in pursuit of design and operating parameters that maximize product yield whilst ensuring that critical quality attributes (CQAs) are kept in specification. Expected outcomes include:
- Reduced number of physical experiments through judicious exploration of the first principles representation of the design space
- Simultaneous optimization of both key process design parameters and critical process operating parameters (CPPs) to optimize yield whilst maintaining CQAs in specification
- Opportunity for CPV during commercial production using digital twin
Establishing a Digital Twin Through a Hybrid Model Workflow
The workflow for establishing a digital twin is outlined in Figure 1 below.
|
|
|
Figure 1: The workflow to establish a digital twin
|
The workflow commences with building a first-principles model that captures the salient mechanisms of the physical process being designed. This first-principles model is then systematically traversed via a virtual design of experiments (DoE) where both the design and operating parameters are perturbed in a comprehensive experimental design to generate simulated data at a unique set of conditions. A multivariate, data-driven model is fit to this simulated data to become the digital clone, or digital twin, of the first-principles model.
This digital twin framework supports several advantageous outcomes:
- Ensure the data-driven model faithfully represents all physical and chemical mechanisms of the first-principles model through the fit to the simulated data
- Easily execute either offline or online (in production) by taking advantage of the low computational burden of the data-driven model
- Rapidly explore and tune all possible combinations of design and operating parameters via modern numerical optimization methods, an endeavor that is much more computationally challenging with the first-principles model
- Drive effective CPV to ensure product yield and quality by readily deploying the digital twin in production to monitor process health
A Practical Example of the Hybrid Modeling Strategy in Pharma
This workflow was applied on chromatography that is widely used in the biopharmaceutical industry for the purification of biomolecules in manufacturing to create a digital twin of a liquid chromatography column, that separates a mixture of the D- and L- enantiomers of the essential amino acid threonine. The digital twin was explored to optimize design parameters such as eluent flow rate, column height and particle radius.
Figure 2 presents the data-driven clone of the first principles model for predicting the CQA (yield of D-threonine). Figure 2A shows the plotted data-driven model coefficients that are used in sensitivity analysis for increased understanding of the relationships between input and output variables. Figure 2B is a trend plot of the simulated model results (Reference) versus the predictions from the data-driven clone based on cross validation (Validation). The plot shows that the two trends track closely together, indicating that the data-driven model is a good fit for capturing the relationships in the first principles model.
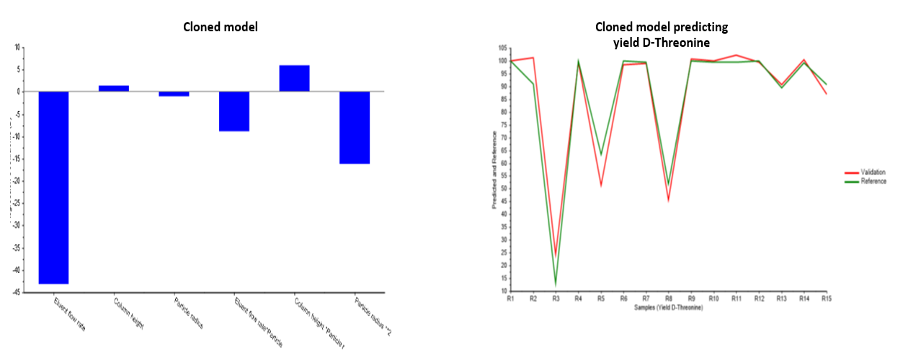 |
| Figure 2A (left) and 2B(right): Model, cloned from first principles, predicting yield of D-threonine
|
This digital twin, the data-driven clone of the first-principles liquid chromatography model, can subsequently be deployed in the regulated production environment as a powerful day-to-day tool for CPV through real-time multivariate monitoring. The application detects and alerts on any deviation in operation from the validated design space, reducing batch discards and mitigating process vulnerabilities, which ultimately saves money.
This liquid chromatography example was presented at the ISPE Biotechnology Conference on June 28-30, 2022, in Boston, MA and again at the APACT Conference on September 14-16, 2022, in Chester, UK.
Further Optimizing the Lifecycle of New Medicines with Digital Twins
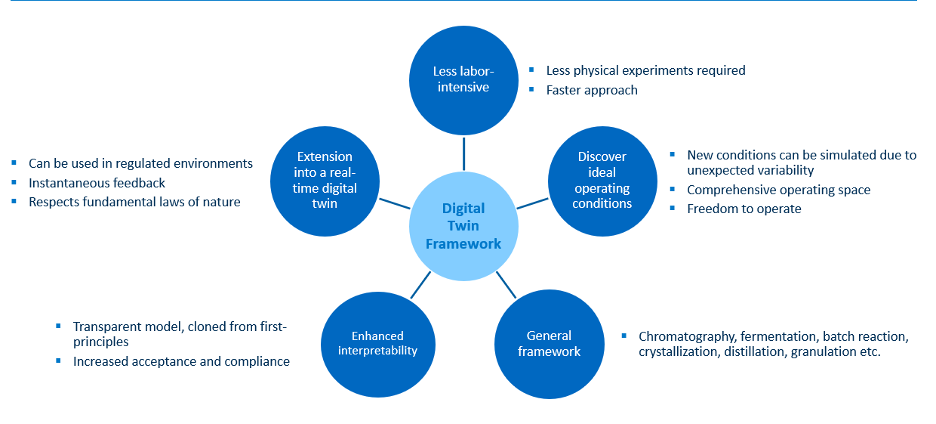
Figure 3 provides a visual summary of the methods and benefits for the digital twin approach discussed in this blog.
|
|
|
Figure 3: Benefits of the digital twin framework
|
The digital twin framework is an important component of a holistic strategy, which extends the offline simulation capabilities based on first-principles models into real-time clones for regulated environments to optimize the lifecycle of new medicines, especially as the pharmaceutical industry transitions from blockbuster to niche medicines. Explore additional digitalization possibilities through this infographic from AspenTech.
1 Rees, Hedley and Plumb, Keith. The Pharmaceutical Industry: Engineering Frustrations. The Chemical Engineer. 28 January 2021
.png?h=250&w=975&la=en&hash=7474A177287C4A487B40FC575A9F1397)




Leave A Comment